Atomic Orbitals
Atomic orbitals depict the movement of electrons around an atom. According to quantum mechanics, no trajectory can be defined. All that can be done is to calculate a region of maximum probability of finding the electrons : the orbitals. They differ according to the energy of the electron. These orbitals were calculated ,and plotted by myself, for the hydrogen atom.
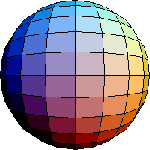
-s orbital
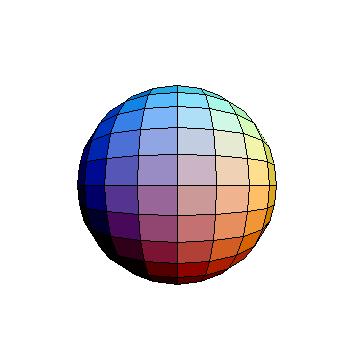
As shown on the atomic models ( Atom I and Atom II), electrons are normally pictured as moving in circular or elliptical orbits around the nucleus. This is a useful model, which can be used to explain various properties (for instance shells and the octet rule). However, electrons don't move in such well defined trajectories. According to quantum mechanics, the most accurate picture is to define orbitals, as regions of probabilities of finding electrons.
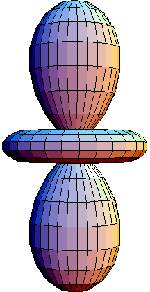
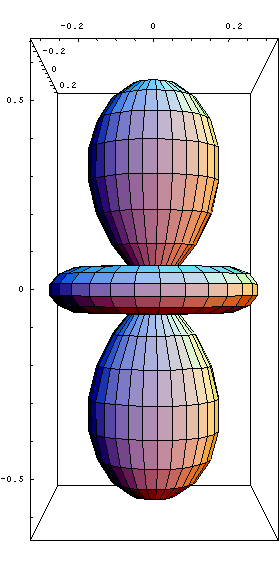
-p orbital
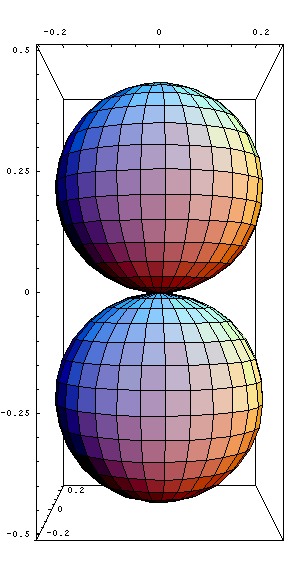
-d orbitals (Look at all 5 d orbitals)
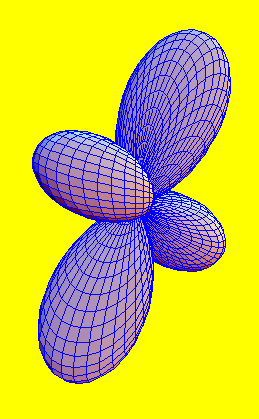
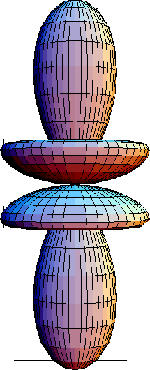
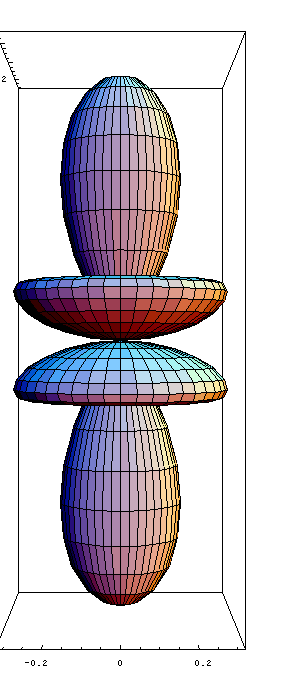
-f orbital
© Ricardo Esplugas. All images in this site can be bought in an enlarged version. Please contact me on ricardochemistry@gmail.com